News
Innovative Medicines based on
ImmunoModulatory Biologics
Sanofi's Phase 2 Success with 'Brivekimig' Targeting TNFα and OX40L Brings a Smile to IMBiologics
-
- Date
- 2025-05-08 15:03:13
-
- View
- 2319
By Yongjun Ji │ Published: April 28, 2025 06:10 │ Updated: April 28, 2025 17:05
-Sanofi shifts HS strategy priority from Amlitelimab to Brivekimig
-TNF and OX40L emerge as key targets for immune modulation as Brivekimig proves PoC
-IMBiologics' dual-antibody candidate 'IMB-101' gains renewed attention

Sanofi, the multinational pharmaceutical company, has signaled a reprioritization of its next-generation autoimmune disease asset to potentially succeed its global top-4 drug by sales, Dupixent (dupilumab). Amlitelimab, a monoclonal antibody targeting OX40L once considered a leading candidate, fell short of expectations. In contrast, Brivekimig—a nanobody-based investigational treatment for hidradenitis suppurativa (HS) that targets both TNF-α and OX40L—met its primary endpoint in a Phase 2 trial and is now emerging as Sanofi’s new strategic asset.
This outcome is particularly noteworthy for Korean biotech IMBiologics, which has licensed out its TNF-α/OX40L bispecific antibody candidate 'IMB-101' to U.S.-based Navigator Medicines in a deal valued at approximately 1.3 trillion KRW. With Sanofi’s positive clinical data, attention is turning toward the potential of this shared target mechanism.
Sanofi Prioritizes Brivekimig Over Amlitelimab in HS Development
On April 24 (local time), Sanofi announced during its Q1 earnings call that Brivekimig had successfully met its primary endpoint in a Phase 2 clinical trial for HS. The candidate demonstrated clinically meaningful improvements in HiSCR50 and other metrics. The safety profile after 28 weeks of treatment aligned with expectations, with no new adverse events observed. Sanofi confirmed it would prioritize Brivekimig over Amlitelimab for HS development going forward.
After the blockbuster success of Dupixent, which generated roughly 21 trillion KRW in revenue last year, Sanofi has aggressively expanded its immunology pipeline to maintain its lead in autoimmune disease treatments. In 2021, it acquired UK-based biotech Kymab for $1.4 billion, securing the rights to Amlitelimab, which targets OX40L. However, in April this year, Amlitelimab failed to meet its primary endpoint in a Phase 2 trial for moderate asthma, falling short of expectations.
Role of TNF-α and OX40L in the Body: A Complex Immune Pathway Beyond Single-Target Modulation
TNF-α (tumor necrosis factor alpha) is a major cytokine involved in inflammatory responses, and its inhibition is a key strategy in treating autoimmune diseases. Globally successful drugs like Humira, Remicade, and Enbrel are TNF-α inhibitors.
OX40L is a co-stimulatory molecule that binds to the OX40 receptor on T cells. It is expressed on the surface of antigen-presenting cells (APCs), while OX40 appears on T cells. Their interaction boosts immune responses in healthy individuals, but in autoimmune patients, it leads to T cell hyperactivation and excessive cytokine release, triggering autoimmune conditions. Targeting both TNF-α and OX40L seeks to modulate complex immune responses that single-target therapies struggle to control.
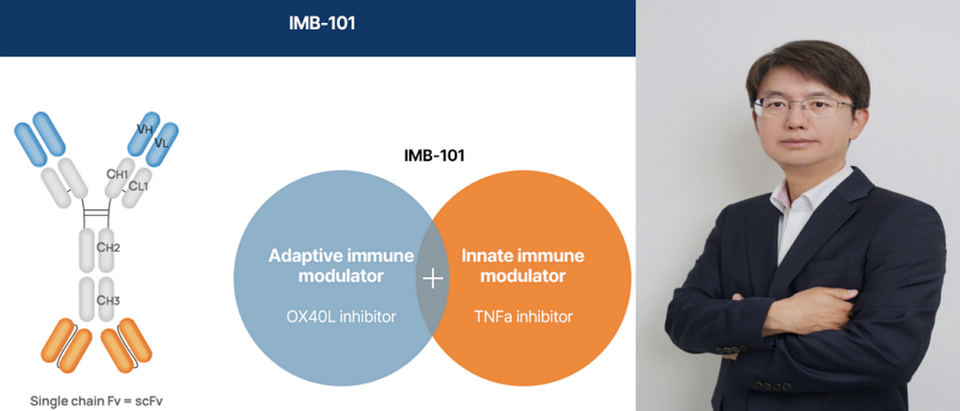
IMBiologics Also Sees a Positive Signal for Its Dual-Antibody Program
Sanofi’s positive clinical data is considered a meaningful signal for IMBiologics, which is developing the bispecific antibody IMB-101 targeting both TNF-α and OX40L. Brivekimig’s success serves as a proof of concept (PoC) for this dual-target approach and may enhance the commercial value of similar pipelines.
IMBiologics had previously licensed out IMB-101 to Navigator Medicines in June 2024 in a 1.3 trillion KRW deal covering global rights excluding China. Navigator has since received approval to begin a Phase 1b global trial for HS using IMB-101. In parallel, Huadong Medicine, the company's Chinese partner, also received approval for a Phase 1b trial in China and is preparing to begin patient enrollment across over ten institutions.
A company representative from IMBiologics commented, “Sanofi’s Brivekimig showing positive Phase 2 indicators in HS gives us reason to expect favorable outcomes from IMB-101, which targets the same molecules. Moreover, as a bispecific antibody, IMB-101 may have advantages in safety and efficacy over Brivekimig, which is a nanobody.”




