News
Innovative Medicines based on
ImmunoModulatory Biologics
IMBiologics Writes 1.7 Trillion KRW Tech Export Success Story — IMB-101's Straightforward Strategy Pays Off
-
- Date
- 2025-05-08 14:56:59
-
- View
- 1815
Robust safety and efficacy data transparently presented at international conferences proves decisive
Signed licensing-out deals worth 1.3 trillion KRW with Navigator Medicines and 438 billion KRW with Huadong Medicine
Secured upfront payments totaling 39 billion KRW, validating commercial viability
By Hyukjin Kwon │ hjkwon@yakup.com
Published: April 23, 2025 06:00 │ Updated: April 23, 2025 16:17
 |
Jung In-soo, Head of Business Operations at IMBiologics, gives a presentation at “ICPI WEEK 2025” held at KINTEX in Goyang, Gyeonggi Province, on April 23. ©Yakup News
“Many people think there’s some special secret to licensing-out success. But in truth, there isn’t. Just one thing: solid data backed by science that proves superior safety and efficacy, and transparent sharing of this data at global conferences to build trust. That was the key driver behind our successful technology transfer.”
Jung In-soo, Head of Business Operations at IMBiologics, shared the background behind the licensing-out success of IMBiologics' bispecific antibody-based autoimmune disease candidate, ‘IMB-101’, during the “1st Pharma/Bio Business Development Strategy Forum 2025,” a side event at “ICPI WEEK 2025,” held at KINTEX, Goyang on April 23.
The forum was hosted by the Pharma/Bio Business Development Society under the Korea Drug Development Research Association (Chairman: Kim Jeong-jin). It served as a platform to discuss strategies for commercializing technology and advancing Korea’s bio-health industry into the global market.
IMBiologics’ ‘IMB-101’ has gained attention as a rare case of a domestic biotech firm achieving back-to-back large-scale licensing deals. The success is attributed to a unique mechanism targeting both immune cell regulator OX40L and inflammatory cytokine TNF, clinically proven safety and efficacy, and a well-structured business development (BD) strategy. IMB-101 is being developed as a treatment for a range of immune diseases including rheumatoid arthritis, atopic dermatitis, and inflammatory bowel disease.
Jung stated, “Publishing IMB-101’s data transparently at major international conferences like the American College of Rheumatology (ACR) laid the groundwork for technology export.” He added, “Navigator Medicines first encountered IMB-101 at the ACR conference in November 2023 and signed the deal within about seven months. Similarly, Huadong Medicine saw the data at BioChina in September 2023 and concluded a deal about 11 months later.”
Such swift technology transfers were made possible by a strategic approach aligned with global standards from the earliest negotiation stages. IMBiologics began by signing Confidential Disclosure Agreements (CDA) to establish information-sharing frameworks. This was followed by preliminary due diligence (DD), exchange of Term Sheets outlining deal terms and rights, and then thorough Index DD to solidify trust necessary for contract finalization.
Jung emphasized, “These deals went beyond mere contracts — they included upfront payments totaling 39 billion KRW, proving real commercial potential. Especially notable, Navigator Medicines began recruiting top talent from global pharma giants like Amgen and GSK to lead IMB-101’s clinical development after the deal was signed.”
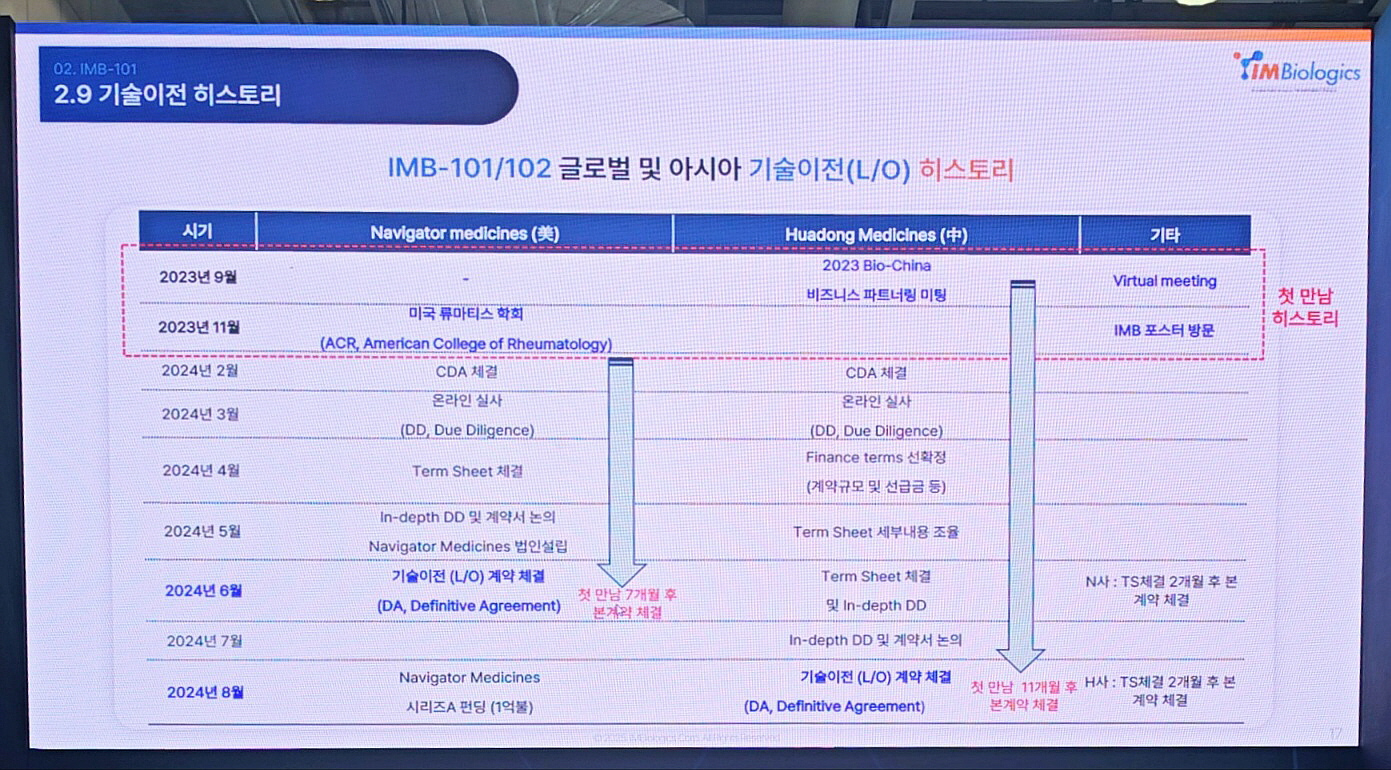 |
The first licensing-out deal for IMB-101 was signed in June 2024 with U.S.-based Navigator Medicines, covering global rights excluding Japan, valued at 1.3 trillion KRW. Of this, about 28 billion KRW was paid upfront. In August of the same year, a second deal worth about 438 billion KRW was signed with China’s Huadong Medicine, covering 40 Asian countries excluding Korea and Japan, with 11.6 billion KRW paid upfront.
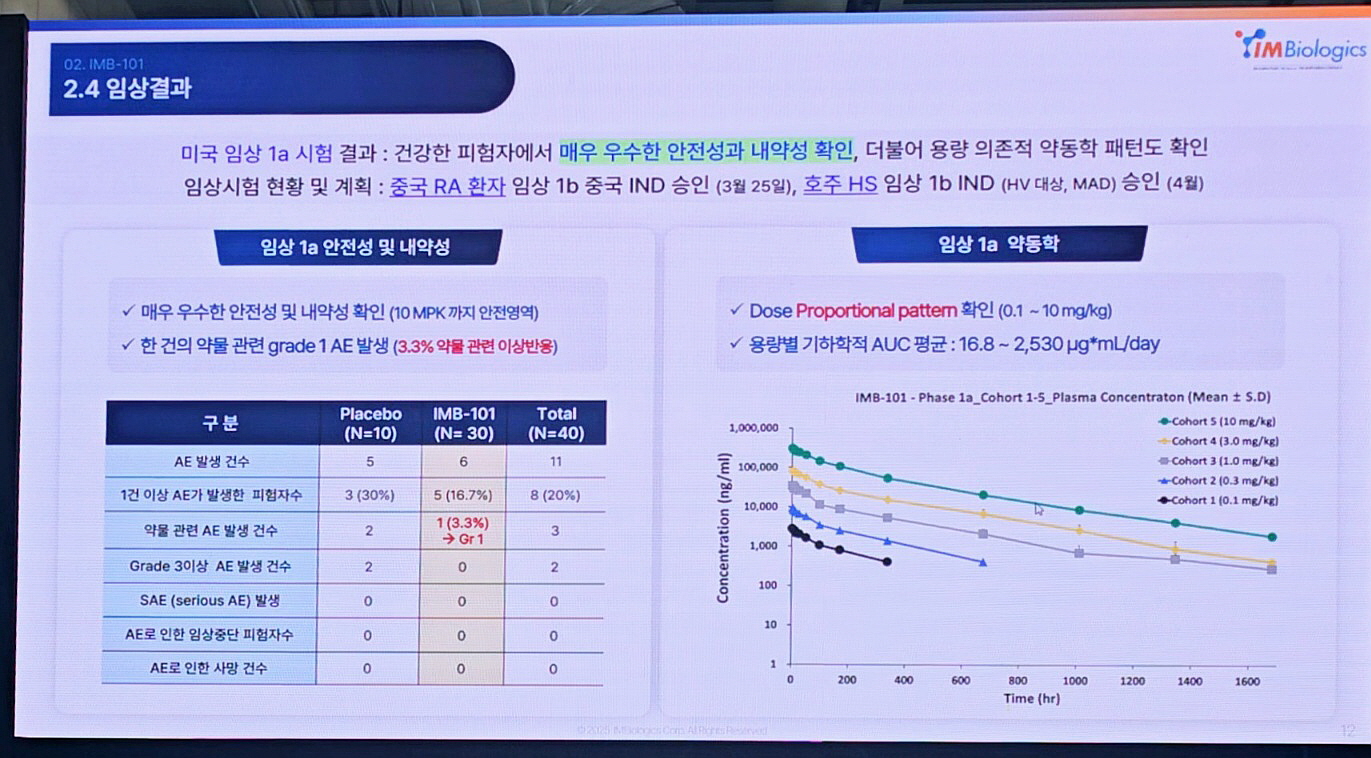
IMB-101’s clinical competitiveness also stands out. IMBiologics submitted its IND application to the FDA in June 2023 and received approval within just one month with no additional requests. Jung explained, “We collaborated with global CROs PPD and Syneos to design a hybrid Phase 1a/1b trial involving healthy adults and patients. In Phase 1a, IMB-101 demonstrated excellent safety and tolerability across 0.1–10mg/kg doses.”
He added, “Only one drug-related adverse event (Grade 1) was observed, and pharmacokinetic analysis confirmed dose proportionality.”
IMB-101 is currently preparing for Phase 1b clinical trials in both the U.S. and China. In preclinical studies, the drug demonstrated meaningful efficacy at one-quarter the dose of AbbVie’s Humira, showing not only anti-inflammatory effects but also suppression of autoantibody production — enhancing its potential as a broad-spectrum immune disease treatment.
Jung added, “IMBiologics is now actively expanding its pipeline beyond autoimmune diseases into immuno-oncology. We are already executing strategies for our second and third licensing deals, aiming to secure global clinical data within 2–3 years and create new business opportunities through collaborations with global pharmaceutical companies.”
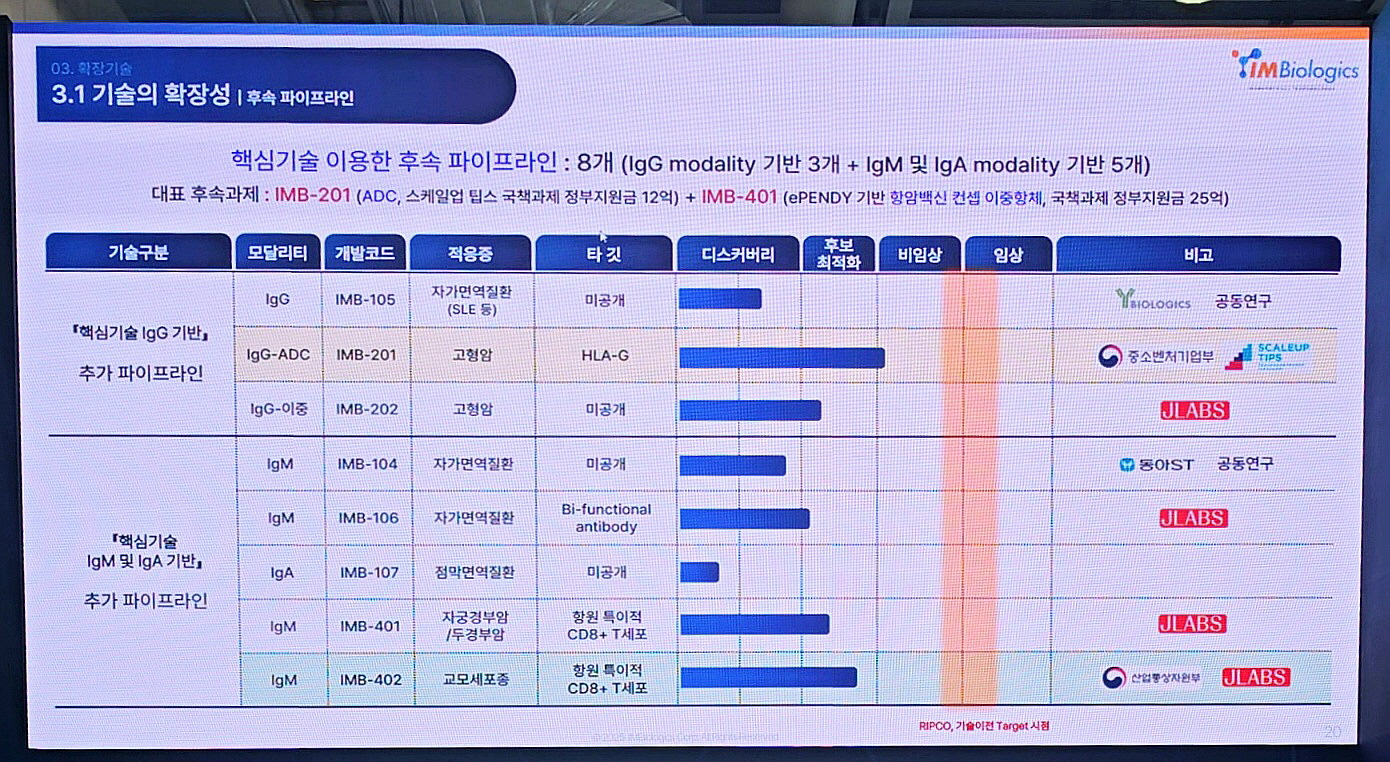
IMBiologics currently holds a pipeline of eight follow-up candidates: three IgG-based modalities and five based on IgM and IgA. Notably, IMB-201 has been selected for the national “Scale-Up Teams” program and has received 1.2 billion KRW in research funding. IMB-401, a bispecific antibody with a cancer vaccine concept, has secured 2.5 billion KRW in government support.
Meanwhile, “ICPI WEEK 2025,” Korea’s largest integrated industry exhibition — now in its 20th year — runs through April 25 at KINTEX Halls 1 and 2 in Goyang. The event showcases the latest technologies and trends across the entire value chain of the pharmaceutical, biotech, and cosmetics industries — from R&D to production, processing, packaging, and logistics. It is co-hosted by Kyungyeon Exhibition and KY Expo, with Yakup News serving as the official media partner.
 |
 |




