The Korea Drug Development Fund (KDDF), a national consortium of three government ministries, is the largest public funder of drug research and development (R&D) in Korea. With a $1.5 billion 10-year budget, KDDF invests more than $150 million a year in over 100 promising projects from academia, biotech, and pharmaceutical companies, spanning the whole R&D process from discovery to clinical trials.
KDDF actively seeks opportunities for co-development and other partnerships with global pharma companies on behalf of Korean biopharma companies to maximize the potential of their assets, and presents three companies with innovative drugs in the immunotherapeutics space working towards these goals.
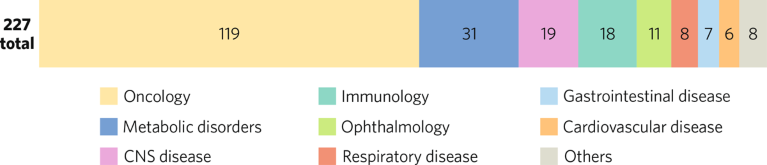
KDDF’s therapeutic pipeline as of December 2022.
IMBiologics
IMBiologics develops innovative new drugs based on immunomodulation and platform technologies. The company concentrates research resources on a two-track strategy: one involves IMB-101, a first-in-class bispecific antibody against tumor necrosis factor-α (TNF-α) and the OX40 receptor ligand (OX40L) that is being developed for the treatment of autoimmune diseases including RA; and the other is a fully proprietary next-generation immunoglobulin M (IgM) platform technology, Enhanced & Engineered Pentamer Body (ePendy).
IMB-101 reduces inflammatory cytokines through inhibition of TNF-α and also normalizes over-active effector immune cells by blocking OX40L to manage chronic inflammation and restore immune balance. IMB-101 has shown superior efficacy compared with TNF-α or OX40L monoclonal antibodies in a collagen-induced arthritis monkey model, even at a quarter molar dose of Humira (adalimumab), and shows robust efficacy without paradoxical reactions that exacerbate inflammation, which are sometimes seen with other biologics. Additionally, IMB-101 reduces osteoclastogenesis, autoantibodies and systemic exposure to TNF antibodies because of localization at the inflammation site.






